
PPT on Reaction Kinetics in Solution | Kinetic Salt Effects
Reaction Kinetics in Solution:
- Molecules in the gas phase occupy ≈0.2 % of the total volume, while molecules in the liquid phase occupy ≈ 50% of the total volume.
- Molecules in the liquid are in permanent contact with each other, and undergo permanent collisions with each other.
Observation on liquid phase reactions:
Except if,
- The reaction occurs with the solvent,
- Strong interactions of the reactant molecule with the solvent (e.g. ionic reactions; → solvent shells),
- The reactions are diffusion controlled.
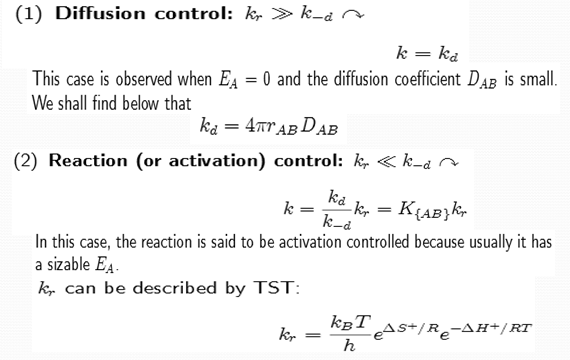
Qualitative model of liquid reactions
However, we can understand the required basis reaction steps as sketched in Fig. which are given below and a no. of limiting cases resulting from these steps.
Structure and dynamics of solution:
 |
| Solvent cages and encounter pairs |
Formal kinetics
Examples
of diffusion-controlled reactions:
The electron-transfer,
Atom-recombination reactions in organic solvent,
The proton-transfer.
The kinetic study of reactions in solution phase is very complicated & is governed by many factors:
- Movement of ions in solution,
- Extent of salvation,
- Nature of reacting species.
These effects are generally known as Salt
Effects and these are of two types-
(a). Primary salt effects.
(b). Secondary salt effects.
Reactions of ions in solution:
The Reactions of ionic species in liquids are one
exception to the rule that reactions in the liquid phase usually have similar rates as in the
gas phase (the other exception being electron transfer reactions). The reason is that
the charged species are very sensitive to their environment, in particular solvation. Changes
of the charge distribution are accompanied by correspondingly large solvation
shell rearrangements.
Effect
of the ionic strength of the solution:
Equilibrium constant for [AB]:
Activity
coefficient from Debye-Hückel theory:
Ionic Strength:
Dependence of the rate constant of the ionic strength:







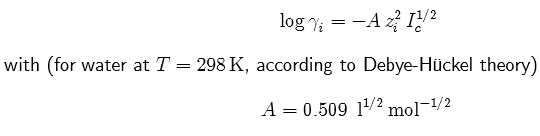




0 Response to "PPT on Reaction Kinetics in Solution | Kinetic Salt Effects"
Post a Comment
Please do not enter any spam link in the comment box.