
Project Assignment on Hydroformylation of Alkenes and Mechanistic Investigation
Wednesday, April 15, 2020
Comment
CONTENT
- Introduction
- Importance of hydroformylation
- Various catalysts employed in hydroformylation reaction
- Cobalt Catalysts for Hydroformylation-
i.
Catalytic cycle using HCo(CO)4
ii.
Disadvantages of cobalt carbonyl complex catalyst
iii.
Phosphine Modified Cobalt Catalysts
iv.
Steric effect of PR3
- Rhodium – Phosphine Catalysts
i.
Catalytic cycle using HRh(CO)(PPh3)3
catalyst
ii.
Drawback of HRh(CO)(PPh3)3
catalyst
- Factors Affecting the n/iso Ratio of Hydroformylation Products
- Water-soluble rhodium catalysts
- Bidendate Phosphine Rh Catalyst
- Other Aspects of Hydroformylation
- Enantioselective Hydroformylation
- Conclusion
- Bibliography
Experimental setup with reactor system
INTRODUCTION
In 1938, Otto Roelen discovered the
hydroformylation – often called the oxo process, one of the first commercially
important homogeneous catalytic reactions. He found that a Cn alkene can be converted to a Cn+1 aldehyde by the addition of H2 and CO to an olefinic double bond catalyzed by
cobalt or rhodium carbonyl complexes.
In simple terms, the equation can be written as
Aldehydes produced by hydroformylation are usually
reduced to alcohols that are used as solvents plasticizers and in the synthesis
of detergents.
IMPORTANCE OF HYDROFORMYLATION
The reaction was first discovered
by Otto Roeln at the Ruhrchemie industry in Germany while studying recycling of
olefins. Since then, the reaction has been developed by industry and account
for more than 7 × 106 tons/year
of aldehydes. It is one of the largest homogeneous catalytic process worldwide.
Actually, aldehydes are not the end product,
they get further reduced to alcohols; sometimes in the same plant or by other
heterogeneous catalysts. The short chain alcohols thus obtained are extensively
used as solvents in the lacquer industry for making plasticisers and the long
chain alcohols are used in the manufacture of synthetic detergents.
As the alkene also has equal
propensity to react with H2, it is interesting to study the thermodynamics
involving both hydrogenation and hydroformylation reactions. The changes are in
entropy and the free energy of these two reactions for propene under standard
conditions are given below.
CH3CH=CH2 + CO + H2
→ CH3CH2CH2CHO
∆H = -150 kJ/mol
CH3CH=CH2 + H2 → CH3CH2CH3
∆H = -126 kJ/mol; ∆G = -88kJ/mol
It becomes evident that the alkane is the
thermodynamically favoured productand not the aldehyde. Since the aldehyde is
the major product, the entropy loss is more important and ∆G becomes less
negative. Also, the reaction is exothermic and if conducted under adiabatic
conditions, the temperature rises and ∆G becomes close to zero.
VARIOUS CATALYSTS EMPLOYED IN HYDROFORMYLATION REACTION:
1) Cobalt Catalyst: HCo(CO)4
2) Cobalt Phosphine-Modified Catalyst: HCo(CO)(PR3)3
3) Rhodium Phosphine Catalyst: HRh(CO)(PPh3)3
4) Aqueous phase Rhodium Catalyst: TPPTS
(Triphenylphosphinetrisulfonate)
5) New generation of Rhodium Catalyst: bidentate
phosphine ligands
Over the years, four catalytic processes have come into
prominence for hydroformylation reaction. These are:
·
The Co2(CO)8
catalysed process,
·
The Co2(CO)8/PR3 catalysed
process,
·
The HRh(CO)(PPh3)3 catalysed
process and
·
The biphasic HRh(CO)(PR3)3 process
(R = m- C6H4SO3Na)
The major difference between these processes are-
The major difference between these processes are-
- The operating temperatures and pressures,
- The ratio of the product formed, n-aldehyde (n/iso ratio),
- The rate of reaction and the control of side reactions such as hydrogenation and
- Ease of recovery of catalyst.
Currently C3
to C15 aldehydes are produced by the oxo process and the are
subsequently converted into amines, carboxylic acids and most importantly to
primary alcohols.
Some of the major industrially important end
products are butanol, 1,4-butanediol ( for THF synthesis), vitamin A and 2-
ethylhexanol. 2-Ethylhexanol is used in making diethylhexyl phthalate
(DEHP),also lnown as dioctyl phthalate(DOP),which is the most widely used
plasticiser in the world.
COBALT CATALYSTS FOR HYDROFORMYLATION:
Cobalt
catalyste dominated the hydroformylation industry till the early 1970s after
which the triarylphosphine rhodium based catalysts took over.The latter are
especially good with C8 or lower alkenes when a higher selectivity
of the linear aldehyde is required. Under H2/CO pressure, cobalt
salts produce HCo(CO)4 as the active catalytic species.
The most widely
accepted mechanism for the catalytic cycle for cobalt based catalyst Co2(CO)8
was proposed by Heck and Breslow in 1961.
Kinetic
studies support a general rate expression as given below:
d[aldehyde]/dt
= k[alkene][Co][pH2][pCO]-1
- Inversely proportional to CO concentration because CO dissociation from the coordinatively saturated 18e- species is required
- Using a 1:1 ratio of H2/CO, the reaction rate is independent of pressure
- HCo(CO)4 is only stable under certain minimum CO partial pressures at a given temperature
- CO pressure ↑ → reaction rate ↓ & high ratio of linear to branched product
- CO pressure ↓ → reaction rate ↑ & branched alkyl ↑ (reverse ß-elminination)
Catalytic cycle using HCo(CO)4:
In the
catalytic cycle, the general catalyst is 16-electron, four coordinate Co1 comples,
HCo(CO)3. This species is not readily available, instead catalytic
precursor Co2(CO)8 is introduced that form HCo(CO)3 under
reaction conditions.
For this
catalytic cycle, Co2(CO)8 and H2 introduced
that form HCo(CO)4( an 18 electron species) which then lossesa CO to
give HCo(CO)3 (a 16 electron species) and creates a vacant coordinatin
site required for alkene.
The alkene coordinates
to this vacant site to form an 18-electron complex whic undergoes migratory
insertion of the olefin into the C-H bond and therefore, another 16-electron
complex having vacant coordination site is formed.
A CO ligand
is then coordinated to the vacant site to form RCH2CH2Co(CO)4
complex. Now insertion of a CO ligand of
RCH2CH2Co(CO)4
occurs into the alkyl-cobalt bond to give the acyl-cobalt complexRCH2CH2CO-Co(CO)3.
The reactant H2 is added oxidatively to the coordinatively
unsaturated cobalt-acyl comlex to give a Co(III) complex which finally
undergoes reductive elimination of product, RCH2CH2CHO
and regenerates the active catalyst HCo(CO)3.
The general
relative reactivity of alkenes for
hydroformylation is as follows:
Disadvantages of cobalt carbonyl complex catalyst:
·
It operates at high temperature (140 – 175oC)
and high pressure (200 – 250 atm.).
- Straight chain(n -) as well as branched chain (iso -) aldehydes are formed. The n:iso ratio is found to be 3:1 which is not a good ratio.The n:iso ratio should be high because straight chain aldehydes are more biodegradable than the branch ones.
Phosphine Modified Cobalt Catalysts:
The addition
of PPh3 ligands to the cobalt carbonyl catalyst brought about a
dramatic change in the rate of the reaction and its regioselectivitydue to electronic and steric effect of substitution of PR3. When a CO is
substituted by the electron donating PR3 group, the back donating
from the metal to the rest of the CO group increases, thereby increasing the
thermal stability of the catalyst against decomposition.If R=Bu, the n/iso
ratio is ( 9:1 ).
Steric effect of PR3:
Bulky PR3 group influences the insertion
direction of alkene to Co complex and geometry of intermediate (favors
Anti-Markovnikov; Hydrogen transferred to carbon with bulkier R group).
The CO
partial pressure required to stabilise the catalyst comes down considerably
from 200–300 to 50–100 bars. Also, the hydridic nature of hydrogen increases as
there is more electron density on the metal. The catalyst can even convert the
aldehyde formed to alcohol by hydrogenation, but the presence of less electron
donating phosphines like PPh3 on the catalyst checks this process
and produces less of the alcohol.
A highly
active catalyst has as additional drawback since it also hydrogenates the alkene
and some alkene is wasted in the formation of unwanted products. Higher stabliity
of the catalyst also means lesser activity.
RHODIUM – PHOSPHINE CATALYSTS:
In 1965,
Osborn, Wilkinson and others reported that Rh(I) catalysts with PPh3
affect not only hydrogenation but also hydroformylation with high
regioselectivity near ambient conditions. As halides were found to be
inhibitors for hydroformylation, the original Wilkinson’s catalyst was modified
to contain no halides. HRh(CO)(PPh3)3 and Rh(acac)(CO)2
are two commonly used catalyst precursors for hydroformylation.
The catalytic
cycle shown that the step are analogous to Heck’s mechanism for
hydroformylation using HCo(CO)4. Kinetic studies on the rhodium
catalyst showed that unlike the cobalt catalyst, there is no inverse dependence
of the rate on CO concentration.
Rate α
[propylene][Rh][pH2]
Drawback of HRh(CO)(PPh3)3 catalyst:
The main
drawback of Rhodium – PPh3 catalyst is a problem related to the
industrial process. Since a high temperature is required for separation of the
long chain aldehyde products, the catalyst decomposes at that temperature. So
the application has been limited to C3
and C4 alkenes.
This problem
was solved by using a water soluble phosphine along with the catalyst and also
by resorting to biphasic catalysis.
Catalytic cycle using HRh(CO)(PPh3)3 Catalyst :
Tables 1 and 2 summaries the
reaction parameters of four catalysts and their advantages and disadvantages
Table 1: Reaction parameters and n/iso
ratio obtained with different catalysts
Catalyst (active form)
|
Reaction parameters
|
(n/iso) ratio maximum
|
Co2(CO)8
[HCo(CO)4]
Co2(CO)8/PR3
R = n-Bu and other similar groups
HRh(CO)(PPh3)3
|
Pressure 200-300 bar
Temp. 110-160oC
Cat. Concentration* 0.1-1.0
Pressure 50-100 bar
Temp. 160-200oC
Cat. Concentration* 0.6
Pressure 15-25 bar
|
3:1
7:1
16:1
|
HRh(CO)(PR3)3
R = mC6H4SO3Na
|
Temp. 80-120oC
Cat. Concentration* 0.01-0.05
Pressure 15-25 bar
Temp. 80-120oC
Cat. Concentration* 0.01-0.05
|
19:1
|
*percentage of
metal/olefin
Table 2: Advantages and disadvantages of
various hydroformylation catalysts
Catalyst (active form)
|
Advantages
|
Disadvantages
|
HCo(CO)4
Co2(CO)8/PR3
R = n-Bu and other similar groups
HRh(CO)(PPh3)3
HRh(CO)(PR3)3
R = mC6H4SO3Na
(water soluble), biphasic catalysis
|
Relatively less alkene hydrogenation(< 2%).
Catalyst decomposition redused due to increased thermal stability of
catalyst.
Better n/iso selectivity due
to increased hydritic character of H.
Low pressure (15–25 bar) aand low temperature.
High n/iso ratio selectivity
(94%)
Easy catalyst recovery and less loss, low catalyst concentration
required.
Less olefin
|
Thermal instability and volatility of (HCo(CO)4 leads to the
deposition of Co or its oxide on the reactor. High pressure of CO (200-300
bar) required to prevent this brings in operational difficulties.
Rate of reaction α 1/[CO].
So, increase in CO pressure reduces rate.
Low n/iso ratio
Pressures and temperatures still on the higher side.
Lower reaction rate (at 180oC, the rate is only 20% of the
rate of HCo(CO)4 operating at 145oC).
Increased hydrogenation of alkenes (up to 15% loss of alkenes).
Good for production of 2-ethylehexanol from propylene (up to 85% yield
in a single reactor).
Applicable only to C3 and C4 olefins as catalyst
is thermally unstable at the high temperatures required for the removal of
products by distillation.
High cost of Rh in comparison to Co.
Low rate of reaction due to reduced miscibility to higher alkenes with
the aqueous phase of catalyst.
Pressure required is on the higher side
|
hydrogenation(< 2 %).
Applicable to long chain olefins as well
|
in comparison to HRh(CO)(PPh3)3.
|
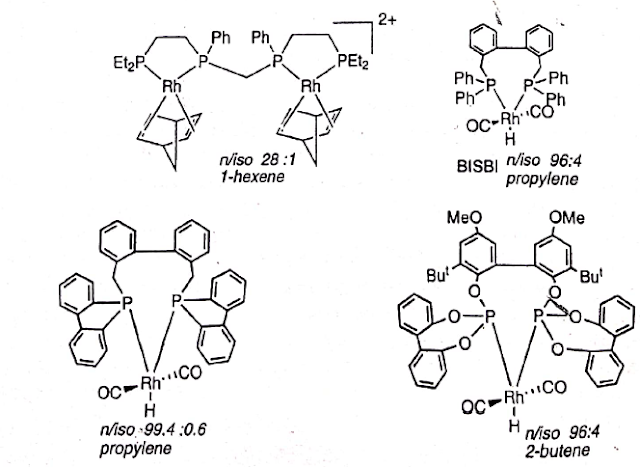
FACTOR AFFECTING THE n/iso RATIO OF HYDROFORMYLATION PRODUCTS:
One of the
directions of recent research in hydroformylationreactions has been to improve
the n/iso
ratio of aldehyde products.The first major development was the discovery of
a chelating biphosphine BISBI developed by Eastman
Kodak..
For example,
Rh with BISBI as ligand gave an n/isp ratioof
96:4 under mild condition. Studies on related biphosphines led to an even
better n/iso ratio; for example, when
PPh3 groups in BISBI were replaced with dibenzophosphole units, it
resulted is an n/isp ratio of
99.4:0.6. However, there was a problem with the catalyst stability.
Piet van Leeuwen and coworkers carried out
systematic studies on a series of biphosphines by varying their bite angle.
They observed that larger natural bite angles in the vicinity of 120o
favoured a higher n/isp ratio of
hydroformylation products.
The use of
phosphites as ligands instead of phosphines also led to a higher n/iso ratio. The percentage of linearity
obtained for PPh3 and P(OPh)3 is very similar at low
ligand concentrations. However, at higher ligand concentrations, phosphites
give a better n/iso ratio of
products. The electronic and steric effects of the substituents on the phosphites
play a significant role in deciding the rate and selectivity of the reactions.
One major finding from a comparative study is that higher the χ value of the
phosphine/phosphite, higher the selectivity towards linear products.
Table 3 gives
some examples indicating this observation. Based on these two findings, a
series of biphosphite ligands having large bite angles were prepared and found
to be useful for realising a high n/iso
ratio of hydroformylation products.
Table 3: Product selectivity in
hydroformylation with rhodium phosphite and phosphine catalysts for 1-heptene
at 90oC temperature and 7 bar pressure of CO/H2.
R3P:R =
|
χ value
|
Linearity of product(%)
|
Ph
PhO
n-Bu
n-BuO
4-Cl-C6H4O
CF3CH2O
|
13
29
4
20
33
39
|
82
86
71
81
93
96
|
WATER-SOLUBLE RHODIUM CATALYSTS:
Ø Water soluble catalyst are made using sulfonated PR3 ligands (3,3′,3″-phosphanetriyltris(benzenesulfonic acid) trisodium salt; TPPTS)
Ø Runs at mild conditions (at 18 bar and 85- 90°C)
Ø Easily separated because water-soluble catalystsremain in aqueous phase and aldehyde is separated into organic phase with higher regioselective ratio between linear and branch.
BIDENDATE PHOSPHINE Rh CATALYSTS
The present invention relates to a bidentate
phosphine ligand, a phosphine phosphorus atom connected by a bridge group, the
bridge group bidentate phosphine ligand containing one ortho position by two
aryl groups fused ring system consisting of, an aryl group which are connected
by two bridges, the first bridge with a -O- or -S- atom composed of a second
bridge containing one oxygen, sulfur, nitrogen, silicon or carbon atom or a
combination of these atoms one group, the two phosphorus atoms linked to two
aryl groups in the ortho -O- or -S- atom of a bridge. This also relates to
bidentate phosphine ligand containing catalyst systems for further one kind of
a transition metal compound, this system can be used for thehydroformylation.
In Rh-catalyzedhydroformylation, the n:iso ratio increases with the bite
angle = (preferred P–M–P angle) of a chelate phosphine, probably because
these ligands facilitate the RE step in the mechanism. The Rh complex (9.27) of
the wide bite angle ligand, BISBI, has proved particularly useful.Ø Over the past 20 years, research was focused on bidentate ligands because of remarkably increased regioselectivity between n/iso ratio of aldehydes.
Ø High regioselectivity is the related to the
stereochemistry of complex combined with the electronic and steric factors of
bidendate PR3.
OTHER
ASPECTS OF HYDROFORMYLATION: The overall effectiveness of other metals are
compared with Co and Rh.
Rh > Co >Ir> Ru >Os>Mn> Fe >
Cr, Mo, W, Ni, Re
Rel. Reactivity: 104-103 1
10-1 10-2 10-3 10-4 10-6< 10-6
ENANTIOSELECTIVE HYDROFORMYLATION:
Enantioselective
hydroformylation is a relatively recent development in hydroformylation
reactions. It is interesting to note that chiral aldehyde will be formed only when
the addition of H2/CO to the alkene occurs in the Markownikoff
manner. In contrast to normal hydroformylation, a better n/iso ratio is preferred in enantioselective hydroformylation as
the n isomer will be nonchiral. Initially,
platinum based catalysts were tried; however, these gave poor n/iso ratio and were plagued by
hydrogenation. The isomerisation of the alkene also occurred.It is interesting
to note that hydroformylation in the Markownikoff sense will form only the chiral
aldehydes.
Rhodium based
chiral catalysts such as HRh(CO)2(R,S)-BINAPHOS have been developed
which give high n/iso ratio as well
as good enantiomeric excess.
CONCLUSION:
· Through the
catalyzedhydroformylation reaction, olefins are converted into aldehydes;
mechanism and corresponding energy calculation were demonstrated.
· The different type of phosphine ligands and
cobalt- and rhodium-based catalysts were introduced; bidendate phosphine Rh
catalyst showed the highest ratios of linear to branched aldehyde even at
ambient conditions.
· Enantio- and
regio-selectivity can be increased if specifically designed ligands on Rh§ catalysts are used
BIBLIOGRAPHY
- B D Gupta and A J Elias, Basic Organometallic Chemistry
: Concepts, Synthesis and Application, Second Edition,Universities Press(
India) Private Limited,2010, 2013, pp 245-252.
- Ajai Kumar, Organometallic and Bioinorganic Chemistry, First
Edition,Aaryush Education, 2014, pp 7-9 to 7-11.
- Robert H. Crabtree, The Organometallic Chemistry of the
Transition Metals, Sixth Edition, John Wiley & Sons, 2014, pp 242-245.
- "Organometallic Chemistry", Spessard and Miessler
- Chem. Rev. 2012, 112, 5675 6 – 5732
- L. H. Slaugh and R. D. Mullineaux11 , J. Organometal. Chem., 1968, 13, 469
THANK
YOU…..!!!











0 Response to "Project Assignment on Hydroformylation of Alkenes and Mechanistic Investigation"
Post a Comment
Please do not enter any spam link in the comment box.